
Molar mass is also known as molecular weight. Molecule is defined as the total sum of the mass of each atom in the molecules Now, on the above hybridization formula, we have to putĬorresponding values to achieve CO2 hybridization. M = Total number of monoatomic atoms bonded to the central atom of valence electrons presence central on the We can also find out hybridization of the molecule directly with Rules, the hybridization of the whole molecule must be decided upon the centralĪtom and carbon is the central atom in CO2 molecule so that CO2 molecule has sp Hybridization because oxygen atom’s steric number is 3 due to two lone pair and So, the steric number of C atom will be 2 and it has sp hybridization. In the case of CO2, The carbon atom has no lone pair of electrons but the C atom is attached to two other Total number of lone pairs + number of atoms directly attached with the atom.Ītoms have sp hybridization, if SN is 3 it will be sp2 hybridization, and so on… Of any atoms based on their steric number (SN) which is given as, And due to these two regionsĪround the central carbon atom, CO2 has sp hybridization.Īccording to the VSEPR theory, we can identify the hybridization Two sigma bonds and no lone pairs of electrons. Regions that are responsible for identifying hybridization. Planer shaped geometry also known as linear geometry’s molecules have alwaysĬO2 has an sp hybridization type. Finally, this is our CO2 Lewis structure diagram.Īs the molecular geometry of the CO2 is linear andĪrrangement like O=C=O which causes the bong angle of CO2 becomes 180°. įrom this, we get no charge on the molecule. The formal charge on each atom can be calculated as,įormal charge (F.C) = Valence electrons (V) – Lone pair of electrons (L) – Bond pair of electrons (B)/2. Here we go each atom have now 8 electrons on its outermost shell which fulfills their octet. So, let’s share two pairs of electrons between both Carbon and Oxygen. Let’s check by sharing one lone pair of electrons but again fail to complete the octet. Now check whether all atoms are completing octet or not on their outer shell, no they aren’t. Place 6 electrons (three lone pairs of electrons) on each carbon atom and 4 electrons (two lone pairs of electrons) on the carbon atom. For this, you must make sure every atom except the central atom must have 8 electrons to follow the octet rule (hydrogen is an exception because it follows duplet rules). It is time to put lone pairs of electrons on atoms. The carbon atom is in the least number so simply it will be the central atom centralized by two oxygen atoms from either side.
#Cf4 molecular geometry how to#
But it can be simply calculated by just dividing the total number of valence electrons by two.įor CO2, total valence electrons are 16 (as calculated in step 1), so total electrons pairs are 16/2= 8.įinding the central atom while drawing a Lewis structure is one of the trickiest parts but as described in how to draw a Lewis structure guide, there is a simple trick for selecting the central atom which is obviously saving extra time and energy. Determine the total number of valence electrons pairsĪs we know the total number of valence electrons are equal to the addition of sigma bonds, pi bonds, and lone pair present at the valence shells. Total number of valence electrons: 4 + 6*2 = 16.Ģ. Here in this molecule, we have one carbon atom and two oxygen atoms. Find out the total number of valence electronsĬO2 is made up of two different atoms: Carbon (C) and Oxygen (O) so first, we should figure out the valence electrons of these two atoms separately. Steps to be followed for drawing CO2 Lewis structureġ.
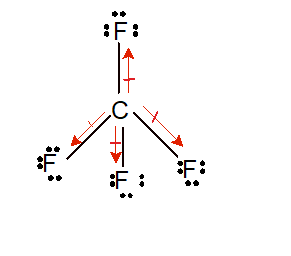
Here both oxygen atoms share two-two electrons with the carbon atom to form two double bonds (O=C) which can also represent by simply placing four dots for a double bond as shown in the above figure.

Both oxygen and carbon atoms need 8 electrons to complete octet in their outermost shells.
It has a direct and very close relationship with human as we emission CO2ĬO2 has a total of 16 valence electrons (carbon has 4 and two oxygen have 12) which are structured as O=C=O. Most commonly it is used as a fire extinguisher in daily With oxygen on both sides where bond length is around 116.3 pm.ĭioxide is widely used in the food industry, oil industry, chemical industry, biological The CO2 molecule is a triatomic molecule in which carbon is covalently double bonded So,ĭioxide which chemical formula is CO2 is a colorless gas found on the earth’sĪtmosphere as a trace gas. Guide you to clear each doubt and misunderstanding for what you are here. Molecular geometry, hybridization, molar mass, and many other aspects of CO2 Article, we are going to learn about carbon dioxide (CO2) Lewis dot structure,


 0 kommentar(er)
0 kommentar(er)
